Abstract
Background: AZA is first line therapy for pts with HR-MDS with an overall response rate (ORR) between 35 and 60% in various publications; with CR/PR rates ranging between 23 and 29% in 3 randomized phase II/III studies (Silverman et al., JCO 2002; Fenaux et al., Lancet Oncol 2009; Sekeres, JCO 2017). AZA also has demonstrated efficacy in older pts with AML (Dombret et al., Blood 2015; Fenaux et al., JCO 2010). Rigosertib interferes with the RAS-binding domains of RAF kinases and inhibits the RAS-RAF-MEK and the PI3Ks pathways (Athuluri-Divakar, Cell 2016). RAS and other genes in this pathway are frequently mutated in HR MDS and putatively drive the malignant clone (Sperling et al., Nat Rev CA 2017). In vitro, the combination of rigosertib with AZA synergistically inhibits growth and induces apoptosis of leukemic cells in a sequence-dependent fashion (Skidan et al; AACR 2006 Abstract 1310).
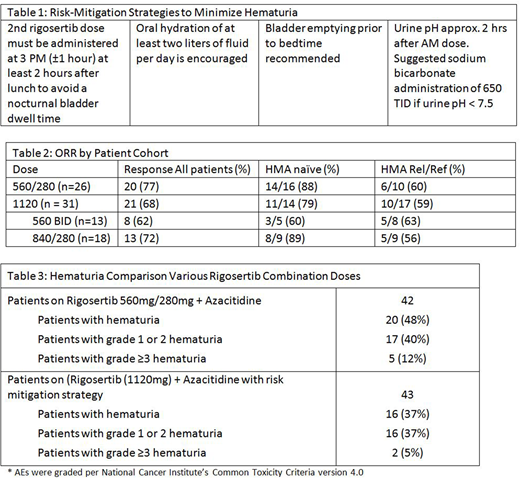
In a trial in lower-risk MDS, oral rigosertib was studied as a single agent at various doses including 560 mg BID for 14-21 days in 28 day cycles and yielded a transfusion independence (TI) rate of 44% - the highest reported TI rate (Raza, ASH Abstract #108660 2017). Based on this observation we expanded the ongoing Phase I/II trial and tested rigosertib at a total of 1120 mg per day with standard dose parenteral AZA (75mg/m2 x 7 days q28 days) in two different schemes as described in methods (NCT01926587). At a dose of 560 mg AM, 280 mg evening the ORR was 77%; 88% for the HMA naïve group and importantly 60% for the HMA Rel/Ref group. Of note, adverse events of interest have been genitourinary (GU) toxicities, particularly hematuria and dysuria. Thus, risk-mitigation strategies (Table 1) were employed to minimize hematuria with higher dose rigosertib at 1120 mg; including the rationale for the lower afternoon (280 mg) dose. We report here the initial results of efficacy and safety.
Methods: In the Phase II Expansion cohort, up to 45 pts with HR MDS/RAEB-t/non-proliferative AML were randomized 1:1 into 2 cohorts both to receive 1120 mg of rigosertib over 24 hours: either 560 mg in the morning and 560 mg in the afternoon, or 840 mg in the morning and 280 mg in the afternoon (part of hematuria/dysuria risk mitigation; moving second dose from evening to afternoon to minimize overnight bladder dwell time as permitted by PK analysis (Maniar, ASH Abstract 2018). Each cohort is stratified among HMA naïve and HMA Rel/Ref pts. Hematologic response is determined per IWG 2006.
Results: As of July 2018 in the rigosertib 1120 mg cohort in combination with AZA, 45 pts were enrolled; 43 treated, 31 evaluable for response; 14 pts continue on treatment, and 31 pts discontinued (includes 2 enrolled but not treated). To be evaluable for response, a minimum of 12 weeks of the doublet was required.
Of the 31 pts evaluable for response, 17 pts are prior Rel/Ref, 14 pts are HMA naïve. The # of prior HMA cycles is 2-15; 13 failed AZA; 1 failed DAC; 2 failed both; and 1 failed other (experimental). The median duration of treatment at this time point for the overall population is 5 months (1-14+). The ORR (Table 2) for the all patients is 68%; 59% for the prior Rel/Ref cohort and 79% for the HMA naïve cohort. For responding patients, responses are seen in both 1120 mg cohorts as shown.
Safety: In 43 patients treated with oral rigosertib at 1120 mg and AZA, with risk-mitigating strategies to minimize hematuria, Grade 1 & 2 hematuria = 16%; ≥3 Grade Hematuria = 5% have been seen to date (Table 3). This compares to an incidence of 12 % Gr 3 hematuria at a lower dose of rigosertib (840 mg); and prior to risk mitigation strategies. Incidence of hematuria of any grade with single agent AZA is 6.3 % & Grade ≥3 2.3% (VIDAZA, package insert 2004).
Conclusion: The combination of oral rigosertib and AZA in HMA naïve patients with HR-MDS is encouraging compared to single agent AZA. The combination also has activity and reverses the HMA clinical resistance in a substantial number of patients after Rel/Ref, a finding with potentially significant clinical implications. Dose exploration with a higher dose of oral rigosertib (1120mg) administered in different dosing schemes in combination with standard dose AZA continues to be studied to optimize safety and efficacy. By employing risk mitigation strategies, the incidence of GU AEs, including hematuria, has been substantially reduced. We will update the safety and efficacy data at the time of presentation. Based on this data a pivotal trial is planned.
Navada:Onconova: Research Funding. Atallah:Pfizer: Consultancy; Abbvie: Consultancy; Jazz: Consultancy; BMS: Consultancy; Novartis: Consultancy. Shammo:Incyte: Consultancy, Honoraria, Research Funding; Onconova: Other: research support; Alexion: Honoraria, Other: research support; Novartis: Consultancy, Honoraria; Celgene: Other: research support. Griffiths:Novartis, Inc.: Research Funding; Celgene, Inc: Honoraria, Research Funding; Astex/Otsuka Pharmaceuticals: Honoraria, Research Funding; Pfizer, Inc.: Research Funding; Alexion Inc.: Honoraria, Research Funding. Khaled:Juno: Other: Travel Funding; Alexion: Consultancy, Speakers Bureau; Daiichi: Consultancy. Pemmaraju:abbvie: Research Funding; stemline: Consultancy, Honoraria, Research Funding; celgene: Consultancy, Honoraria; Affymetrix: Research Funding; samus: Research Funding; SagerStrong Foundation: Research Funding; cellectis: Research Funding; daiichi sankyo: Research Funding; novartis: Research Funding; plexxikon: Research Funding. Zbyszewski:Onconova Therapeutics, Inc: Employment, Equity Ownership. Maniar:Onconova Therapeutics, Inc: Employment, Equity Ownership. Petrone:Onconova Terapeutics Inc.: Employment, Equity Ownership. Fruchtman:Onconova Therapeutic Inc: Employment, Equity Ownership. Silverman:Johnson and Johnson: Research Funding; Onconova Therapeutics Inc.: Patents & Royalties, Research Funding; Bayer: Research Funding; Celgene: Research Funding; Medimmune: Research Funding; Mount Sinai School of Medicine: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal